
Many sellers and agencies have probably had Safety Data Sheet (SDS), Exemption Sheet, or Product Compliance documentation requested for products at least once. This article will walk you through what these documents are, and their impact on seller listings and accounts.
Safety Data Sheet (SDS)
A Safety Data Sheet (SDS) is a detailed instruction guide on safely handling, storing, and labeling specific goods. The SDS includes various data such as product names, labels, substances, first-aid directions, hazard information, protective gear, storage advice, toxicity, and disposal.
Where and how to get AN SDS
Commonly, it is up to the manufacturer to provide an SDS, but many contract manufacturers in China and other Asian countries don’t know how to issue and send one. The problem with Safety Data Sheets is differences in standardized templates according to the products’ country, region, and categories. Because of that, many distributors, sellers, and wholesalers are reaching out to specialized third-party agencies handling all the SDS creation or updating procedures.

Amazon Dangerous Goods Review Process
Fulfillment by Amazon (FBA) service handles hazardous goods. The dangerous goods team ensures that all FBA products meet Amazon’s safety standards. The team works with the seller’s catalog items to ensure that hazmat products are handled correctly, and they check the following catalog information to ensure it is accurate and complete: product name, detailed description, bullet points, and images. If catalog information is altered, you must undergo a second review, and reclassification of the product may also be required. Furthermore, battery-operated items and products free of hazardous chemicals require a Safety Data Sheet.
The process of an SDS or Exemption Sheet Submission
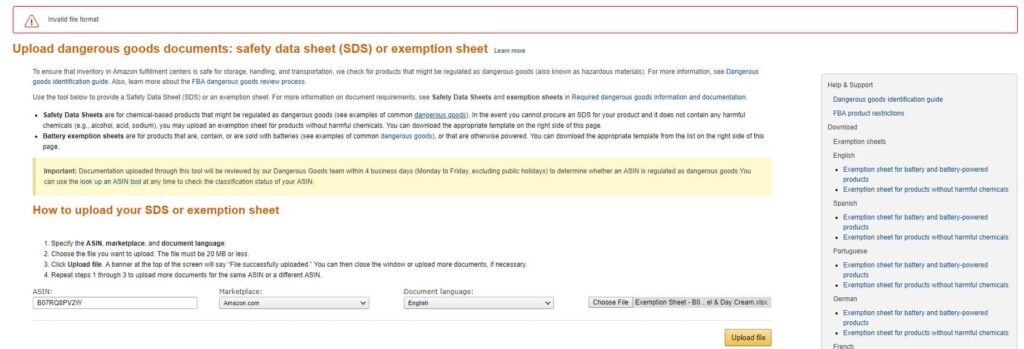
Hazmat reviews can be sped up by providing a valid SDS from the outset, because Safety Data Sheets state whether a product contains hazmat materials, such as battery-powered products or chemical-based goods. If you cannot get an SDS, you can upload an Exemption Sheet if the material does not include harmful chemicals. To do so:
Create a template for the Exemption Sheet.
- Fill in the relevant fields, such as ASIN and warning label acknowledgments.
- Select the ASIN, marketplace, and language.
- Choose the Exemption Sheet (it must be less than 20 MB).
- Click on ‘Upload file’.
- Repeat the process if you need to submit more sheets.
The dangerous goods team usually performs the review process within four days.
Amazon Product Compliance Documentation Requests
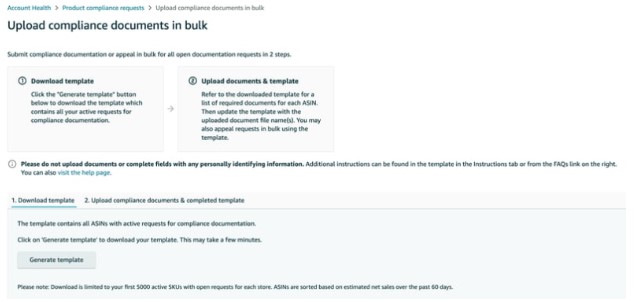
Amazon may request test reports, label photocopies, and product certificates to verify that a product is safe and fully compliant with national regulations in the relevant market. Such a review can be triggered when listing a new product, receiving consumer complaints, or by Amazon’s internal compliance review team, who often requests these documents.
Amazon requests the necessary documents to verify that the item complies with the appropriate standards and regulations. The documents needed to depend on the product category in question but typically include test reports and certifications. In most cases, Amazon requires lab test reports and other compliance documents, and self-issued product certificates or declarations may also be required. Amazon may request photocopied labels to evaluate whether the product is properly labeled with CE marking, country of origin, and more.
The compliance team will also check the following:
- Is the test report/certificate appropriate for the same product?
- Is the test report/certificate held by the right company?
- Is the test report/certificate adequately formatted?
- Is all necessary data included in the test report/certificate?
- Is the label correct?
Some of BellaVix’s Experiences
At BellaVix, we had multiple cases for our clients where we needed to provide SDS, Exemption Sheets, and Product Compliance documentation.
For our client selling supplements, the Amazon product compliance team requested documentation for two of their products. From this experience, we have concluded that for such categories, the documentation requested would be:
- Valid GMP (Good Manufacturing Practices) certificate.
- Images of all sides of the product, including the top and bottom.
- COA (Certificate of Analysis) for the product from the certified laboratory accepted by Amazon. The COA requirement might need to contain additional specific ingredient analysis depending on what product you want to sell or are selling.
Another client, selling undergarments, had some of their products determined as menstrual devices, and according to Amazon requirements, all such products needed to comply with the standards and regulations listed below:
- 21 CFR Part 807 (FDA Establishment Registration and Device Listing);
- 21 CFR 807 Subpart E (FDA 510(k) Premarket Notification); and
- 21 CFR Part 801 (Medical Device Labeling).
The documentation requested was:
- Documentation demonstrating the following:
- FDA Establishment Registration number (21 CFR Part 807) – screenshot
- FDA Device Listing (21 CFR 807) – screenshot
- FDA 510(k) Premarket Notification (21 CFR 807 Subpart E) – FDA Approval Letter
- Test Reports
- Some labs provide special Amazon rates for testing. To find more information about these providers, visit the Amazon Service Provider Network.
- Product Packaging and Label Images
- Product images must show all sides of the product packaging.
Key Takeaways
- Safety Data Sheets must be submitted on Amazon for products determined as dangerous goods (hazmat).
- If it is not possible to get an SDS, and the item does not contain harmful chemicals, then you can upload an Exemption Sheet.
- Amazon sometimes contacts sellers to request test reports, label photocopies, and product certificates to verify that the product is safe and fully compliant with the national regulations in the concerned marketplace.
- In case of product compliance requests:
- Documentation requested may vary according to the product type.
- All requested documentation must be submitted to ensure your products are approved/relisted.
- The requested certification must be valid if they have expiration dates and must be renewed on time; otherwise, sellers will face the same situation whenever the certificate expires. Planning accordingly in these cases is essential.
- Attempting to provide false certificates or certificates by labs not accepted by Amazon will not give any positive results and can negatively impact the seller’s account.
- Failing to provide all the requested information in COAs will result in resubmissions or possible permanent removal of the listing.
- The best way to speed up the process is to use Amazon recommendations for labs and follow all the instructions. In any other case, sellers are risking being declined and repeated submissions.
- The only way to start selling products again once your listings have been suspended due to compliance documentation requests or dangerous goods information is by submitting the requested documents (valid documents).
- Deleting the listing with required documentation (any documentation) and attempting to relist the same product without documentation is against Amazon ToS and can result in the permanent removal of seller privileges.
If you have additional questions or want us to help you on your Amazon journey, don’t hesitate to contact the BellaVix Team.
Keep up with the latest Amazon and Walmart news updates and subscribe to our BellaVix newsletter 👇👇👇
